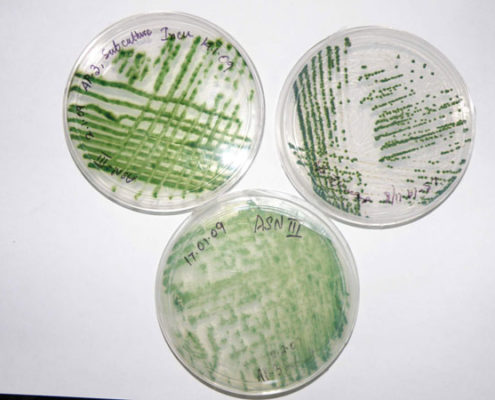
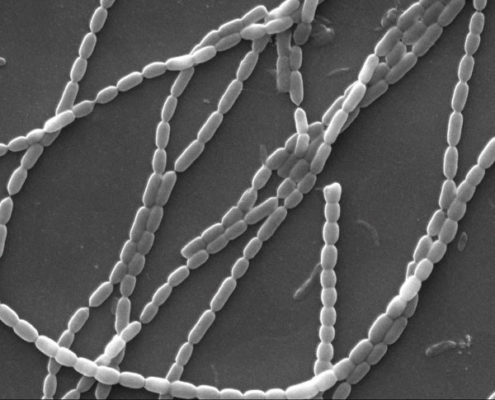
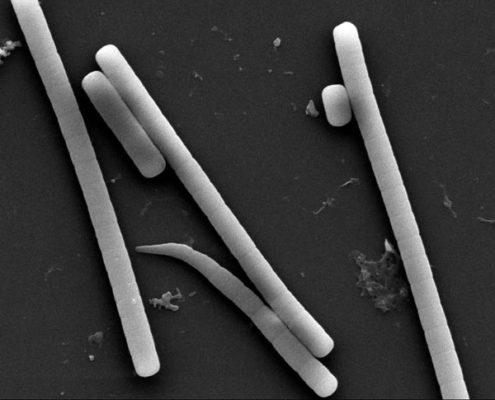
Eight obligately halophilic, euryhaline cyanobacteria from intertidal soil of the Sundarbans were isolated in artificial sea-water nutrients (ASN-III) medium. Antimicrobial activity, 16S rRNA gene sequences, phenotypic characters as well as growth and antibiosis in response to variable salinity, temperature, phosphate concentration, and pH were studied. Minimum inhibitory concentrations (MIC) of the extracts against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, and multiple drug-resistant clinical isolates ranged between 0.25 and 0.5 mg/ml. Cytotoxicity tests showed 73%–84% human colon adenocarcinoma (HT-29 ⁄ C1) cell survival at MIC values, indicating that the extracts were nontoxic. Morphologically, six cyanobacteria were assigned to the Lyngbya-Phormidium-Plectonema (LPP) group-B, and one each was assigned to Oscillatoria and Synechocystis genera. Glycerol, mannitol, and starch supported better photoheterotrophic growth than simpler mono- and disaccharides. No heterocyst formation was observed when grown under nitrogen-starved conditions. All isolates survived 7 ppt salinity, grew at minimum 32 ppt salinity, and showed sustained growth throughout 32 ppt–82 ppt salinity but matured poorly in freshwater medium supplemented with 30.0 g/l NaCl. Antimicrobial production occurred only at 32 ppt salinity. While four of the eight isolates demonstrated sustained growth at 37ºC, maximum antimicrobial activity was obtained at 25ºC. All strains showed maximum growth and antimicrobial elaboration at 0.04 g/l phosphate. All isolates thrived at pH 9.5; six grew at pH 4.5, though antimicrobial production occurred only at pH 7.5. Molecular phylogenetic analysis based on 16S rRNA gene sequences of the filamentous isolates validated the previous taxonomic affiliations established on morphological characteristics. This is the first study of antimicrobial-producing halophilic cyanobacteria from the mangroves.
A novel approach was employed to study the growth of three cyanobacterial strains namely Oscillatoria sp. (AP17), Leptolyngbya sp. (AP3b) and Chroococcus sp. (AP3U) . Furthermore, their broad metabolite profile, production of pigments, exopolysaccharide (EPS) and antimicrobial activity were evaluated in response to contrasting cultivation modes: biofilm or planktonic. The biofilm culture mode was carried out in the patented conico-cylindrical flask (CCF) and the planktonic culture mode was carried out in an Erlenmeyer flask (EF). The amount of polysaccharide that was released and that remained capsular/bound was higher in CCF compared to EF cultivation. Amount of chlorophyll a produced by Oscillatoria (AP17) was higher in the CCF compared to the EF cultivation. Highest antimicrobial activities were exhibited by Leptolyngbya (AP3b) biofilm than other biofilms as well as planktonic biomass. Metabolite profiles of Cyanobacteria were revealed by various chromatographic techniques and showed clear differences among the two contrasting modes of cultivation. The results showed clear differences in the mode of growth for achieving maximum chlorophyll a, EPS and bioactive metabolite production of the Cyanobacteria. The present study augmented the information which can enhance wider exploration of the biofilm mode of cultivation of Cyanobacteria.